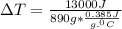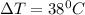Chemistry, 05.07.2019 18:00 calebmoore925
A1.1 kg block of iron at 38 ∘c is rapidly heated by a torch such that 13 kj is transferred to it. what temperature would the block of iron reach assuming the complete transfer of heat and no loss to the surroundings? if the same amount of heat was quickly transferred to a 890 g pellet of copper at 38 ∘c, what temperature would it reach before losing heat to the surroundings? cs, fe(s)= 0.450 j/g*c cs, cu(s)= 0.385 j/g*c

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

You know the right answer?
A1.1 kg block of iron at 38 ∘c is rapidly heated by a torch such that 13 kj is transferred to it. wh...
Questions


History, 27.07.2019 03:50

Health, 27.07.2019 03:50



History, 27.07.2019 03:50

History, 27.07.2019 03:50



Mathematics, 27.07.2019 04:00





Social Studies, 27.07.2019 04:00





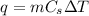
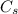 is specific heat and
is specific heat and  is the change in temperature.
is the change in temperature.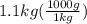 = 1100 g
= 1100 g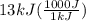 = 13000 J
= 13000 J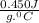
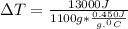
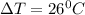
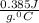 .
.