

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
In standardizing the solution of aqueous sodium hydroxide, a chemist overshoots the end point and ad...
Questions

Biology, 28.05.2020 00:03

Spanish, 28.05.2020 00:03


Chemistry, 28.05.2020 00:03

Mathematics, 28.05.2020 00:03

Mathematics, 28.05.2020 00:03


Computers and Technology, 28.05.2020 00:03





History, 28.05.2020 00:57


Mathematics, 28.05.2020 00:57

History, 28.05.2020 00:57



Mathematics, 28.05.2020 00:57

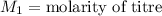 ,
, 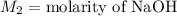
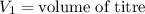 ,
, 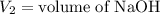
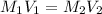
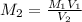 ....(1)
....(1)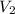 .
.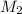 in equation (1), will get lowered , which means that the concentration of NaOH was lower than that of the actual value. Hence underestimated concentration of NaOH.
in equation (1), will get lowered , which means that the concentration of NaOH was lower than that of the actual value. Hence underestimated concentration of NaOH. 


