
Chemistry, 05.07.2019 11:00 Ashley606hernandez
An 8.65 g sample of an unknown group 2a metal hydroxide is dissolved in 85.0 ml of water and titrated with 2.50 m hcl(aq). if it takes 56.9 ml of the acid to reach the end point of the titration (a) what is the molar mass of the metal hydroxide? (b) which of the following is in the metal hydroxide: ca2+, sr2+, ba2+?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1



Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
An 8.65 g sample of an unknown group 2a metal hydroxide is dissolved in 85.0 ml of water and titrate...
Questions

Geography, 02.02.2020 18:52

Mathematics, 02.02.2020 18:52

Biology, 02.02.2020 18:52

Mathematics, 02.02.2020 18:52



Social Studies, 02.02.2020 18:52





World Languages, 02.02.2020 18:52

Biology, 02.02.2020 18:52





Mathematics, 02.02.2020 18:52

History, 02.02.2020 18:52

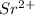 .
.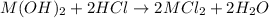
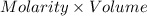
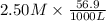
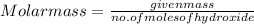
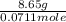
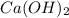 = (40.078 + 34) g/mol = 74.093 g/mol
= (40.078 + 34) g/mol = 74.093 g/mol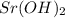 = (87.66 + 34) g/mol = 121.66 g/mol
= (87.66 + 34) g/mol = 121.66 g/mol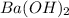 = (137.32 + 34) g/mol = 171.32 g/mol
= (137.32 + 34) g/mol = 171.32 g/mol

