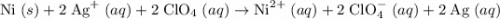Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1


Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Determine the oxidizing agent in the following reaction. ni(s) + 2 agclo4(aq) → ni(clo4)2(aq) + 2 ag...
Questions

Mathematics, 22.09.2020 23:01

Computers and Technology, 22.09.2020 23:01

Chemistry, 22.09.2020 23:01




Mathematics, 22.09.2020 23:01


Mathematics, 22.09.2020 23:01

Biology, 22.09.2020 23:01

Mathematics, 22.09.2020 23:01

Chemistry, 22.09.2020 23:01

English, 22.09.2020 23:01

Chemistry, 22.09.2020 23:01



Mathematics, 22.09.2020 23:01


Mathematics, 22.09.2020 23:01

Chemistry, 22.09.2020 23:01

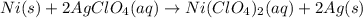
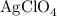 serves as the oxidizing agent in this reaction.
serves as the oxidizing agent in this reaction.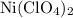 in the products exists as ionic salt dissolved in an aqueous solution. Rewriting the chemical equation as an ionic one might help eliminate spectator ions and make changes in oxidation states more apparent.
in the products exists as ionic salt dissolved in an aqueous solution. Rewriting the chemical equation as an ionic one might help eliminate spectator ions and make changes in oxidation states more apparent.