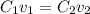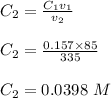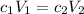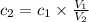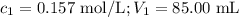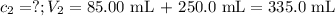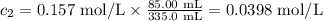Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Calculate the molarity of a solution made by adding 250.0 ml of water to 85.00 ml of a 0.157 m solut...
Questions

Mathematics, 05.02.2021 20:20

Mathematics, 05.02.2021 20:20


English, 05.02.2021 20:20




Social Studies, 05.02.2021 20:20

Mathematics, 05.02.2021 20:20

Mathematics, 05.02.2021 20:20




Mathematics, 05.02.2021 20:20

Mathematics, 05.02.2021 20:20

Physics, 05.02.2021 20:20


Mathematics, 05.02.2021 20:20

Health, 05.02.2021 20:20