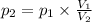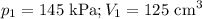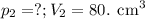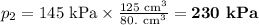Chemistry, 05.07.2019 02:00 cairolove228
At 25°c, gas in a rigid cylinder with a movable piston has a volume of 145 ml and a pressure of 125 kpa. then the gas is compressed to a volume of 80. ml. what is the new pressure of the gas if the temperature is held at 25°c? (1) 69 kpa (3) 160 kpa (2) 93 kpa (4) 230 kpa the first person didn't make sense as p1v1 = p2v2 isnt the same thing as p1v1/v2 that literally doesn't make sense, so could someone me !

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 23.06.2019 08:30
Explain how to convert from one unit to another in the metric system.
Answers: 3

Chemistry, 23.06.2019 10:10
Which orbitals form a pi bond? a. the s orbital and three p orbitals b. the s orbital and two p orbitals c. overlapping p orbitals d. overlapping hybrid orbitals
Answers: 2

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3
You know the right answer?
At 25°c, gas in a rigid cylinder with a movable piston has a volume of 145 ml and a pressure of 125...
Questions










Social Studies, 21.04.2020 03:40

Mathematics, 21.04.2020 03:40









Computers and Technology, 21.04.2020 03:45

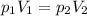
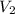 .
.