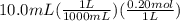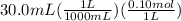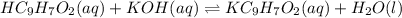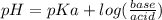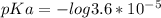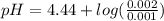Chemistry, 04.07.2019 23:00 dnprops1544
What is the ph when 10.0 ml of 0.20 m potassium hydroxide is added to 30.0 ml of 0.10 m cinnamic acid, hc9h7o2 (ka = 3.6 × 10–5) 4.74 9.26 1.60 12.40

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1


You know the right answer?
What is the ph when 10.0 ml of 0.20 m potassium hydroxide is added to 30.0 ml of 0.10 m cinnamic aci...
Questions


Mathematics, 10.10.2020 20:01


Business, 10.10.2020 20:01



Social Studies, 10.10.2020 20:01



English, 10.10.2020 20:01


Mathematics, 10.10.2020 20:01







Mathematics, 10.10.2020 20:01