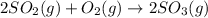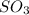How would adding the catalyst nitrogen monoxide (no) affect this reaction? 2so2(g) + o2(g) → 2so3(g) a. no increases the rate at which so3 molecules are formed. b. no reacts with so3 to produce more so2 molecules. c. no decreases collisions between the so2 and o2 molecules. d. no increases the concentration of the so2 and o2 molecules. e. no increases the activation energy of the so2 and o2 molecules.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2
You know the right answer?
How would adding the catalyst nitrogen monoxide (no) affect this reaction? 2so2(g) + o2(g) → 2so3(g...
Questions

Business, 25.11.2020 18:00

Mathematics, 25.11.2020 18:00


Physics, 25.11.2020 18:00


Mathematics, 25.11.2020 18:00




Mathematics, 25.11.2020 18:00

Mathematics, 25.11.2020 18:00

English, 25.11.2020 18:00

Mathematics, 25.11.2020 18:00


Mathematics, 25.11.2020 18:00


Computers and Technology, 25.11.2020 18:00

Mathematics, 25.11.2020 18:00


Social Studies, 25.11.2020 18:00