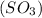Chemistry, 04.07.2019 22:00 masaiyahrhemanow
How would adding the catalyst nitrogen monoxide (no) affect this reaction? 2so2(g) + o2(g) → 2so3(g) a. no increases the rate at which so3 molecules are formed. b. no reacts with so3 to produce more so2 molecules. c. no decreases collisions between the so2 and o2 molecules. d. no increases the concentration of the so2 and o2 molecules. e. no increases the activation energy of the so2 and o2 molecules.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1


Chemistry, 23.06.2019 10:20
Determine the mass of the object below with accuracy and to the correct degree of precision. a. 324.2 g b. 324 g c. 324.30 g d. 324.25 g
Answers: 3
You know the right answer?
How would adding the catalyst nitrogen monoxide (no) affect this reaction? 2so2(g) + o2(g) → 2so3(g...
Questions




Mathematics, 31.05.2021 18:50

Mathematics, 31.05.2021 18:50

English, 31.05.2021 18:50

English, 31.05.2021 18:50

Physics, 31.05.2021 18:50

Computers and Technology, 31.05.2021 18:50


Mathematics, 31.05.2021 18:50


Mathematics, 31.05.2021 18:50


Mathematics, 31.05.2021 18:50

Mathematics, 31.05.2021 18:50



Physics, 31.05.2021 18:50

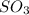 molecules are formed.
molecules are formed.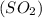 and
and 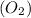 undergo collision and can cross the energy barrier and convert to products
undergo collision and can cross the energy barrier and convert to products