
Chemistry, 04.07.2019 22:00 jlayne0605
Which substance in this redox reaction is the oxidizing agent? cu + 2agno3 → 2ag + cu(no3)2 a. n b. agno3 c. cu d. no3− e. cu(no3)2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
Which substance in this redox reaction is the oxidizing agent? cu + 2agno3 → 2ag + cu(no3)2 a. n b...
Questions





History, 08.02.2021 22:00

Computers and Technology, 08.02.2021 22:00

Mathematics, 08.02.2021 22:00




Biology, 08.02.2021 22:00

History, 08.02.2021 22:00

English, 08.02.2021 22:00

Mathematics, 08.02.2021 22:00

Mathematics, 08.02.2021 22:00

Mathematics, 08.02.2021 22:00



Biology, 08.02.2021 22:00

Mathematics, 08.02.2021 22:00

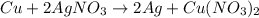
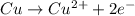
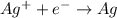
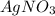 is considered as an oxidizing agent and therefore the correct answer is Option B.
is considered as an oxidizing agent and therefore the correct answer is Option B.

