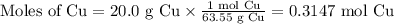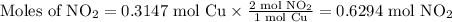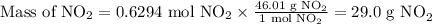Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1


Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Cu(s) + 4hno3(aq)= cu(no3)(aq)+ 2no2(g) + 2h2o(l) how much no2 gas would be formed by the reaction...
Questions

English, 24.06.2019 00:00


Mathematics, 24.06.2019 00:00


English, 24.06.2019 00:00

Mathematics, 24.06.2019 00:00



Mathematics, 24.06.2019 00:00


Mathematics, 24.06.2019 00:00



Biology, 24.06.2019 00:00






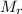 : 63.55 46.01
: 63.55 46.01