
Chemistry, 04.07.2019 20:30 kingje1477
Calculate the standard enthalpy of combustion. the standard enthalpy of formation of sucrose is - 2226.1kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2
You know the right answer?
Calculate the standard enthalpy of combustion. the standard enthalpy of formation of sucrose is - 22...
Questions

Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

History, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

Social Studies, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

English, 11.11.2020 01:00

History, 11.11.2020 01:00


Mathematics, 11.11.2020 01:00


Computers and Technology, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

Spanish, 11.11.2020 01:00

English, 11.11.2020 01:00


Biology, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00


Business, 11.11.2020 01:00

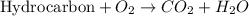
![\Delta H^o_{rxn}=\sum [n\times \Delta H_f^o_{(product)}]-\sum [n\times \Delta H_f^o_{(reactant)]}](/tpl/images/0051/4711/84379.png)
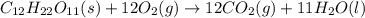
![\Delta H^o_{rxn}=[(12\times \Delta H_f^o_{(CO_2)})+(11\times \Delta H_f^o_{(H_2O)})]-[(1\times \Delta H_f^o_{(C_{12}H_{22}O_{11})})+(12\times \Delta H_f^o_{(O_2)})]](/tpl/images/0051/4711/fac1e.png)
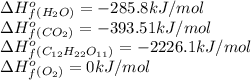
![\Delta H^o_{rxn}=[(12\times (-393.51))+(11\times (-285.8))]-[(1\times (-2226.1))+(12\times (0))]\\\\\Delta H^o_{rxn}=-5636.52kJ](/tpl/images/0051/4711/5e149.png)


