
Chemistry, 04.07.2019 10:00 kenken2583
A50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorimeter filled with water. the calorimeter and the water have a combined mass of 250.0 g and an overall specific heat of 1.035 cal/g•°c. the initial temperature of the calorimeter is 10.00°c. the system reaches a final temperature of 11.08°c when the metal is added. any heat lost by the metal is

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

You know the right answer?
A50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorime...
Questions

Mathematics, 13.03.2021 03:20



History, 13.03.2021 03:20




Chemistry, 13.03.2021 03:20



Social Studies, 13.03.2021 03:20

Mathematics, 13.03.2021 03:20

Mathematics, 13.03.2021 03:20

Mathematics, 13.03.2021 03:20

Mathematics, 13.03.2021 03:20


Mathematics, 13.03.2021 03:20

Mathematics, 13.03.2021 03:20

Physics, 13.03.2021 03:20

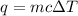 .
. is change in temperature.
is change in temperature.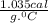 .
. 

