

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
Adding naoh to an aqueous solution containing ni2+ results in the precipitation ofni(oh)2. the stand...
Questions


Spanish, 19.04.2021 04:30

Mathematics, 19.04.2021 04:30

Mathematics, 19.04.2021 04:30

Mathematics, 19.04.2021 04:30



Mathematics, 19.04.2021 04:30

Mathematics, 19.04.2021 04:30

Mathematics, 19.04.2021 04:30

Mathematics, 19.04.2021 04:30

Biology, 19.04.2021 04:30


Physics, 19.04.2021 04:30


English, 19.04.2021 04:30



History, 19.04.2021 04:30

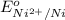 = +0.25 V
= +0.25 V![K_{sp}=[Ni^{2+}][OH^-]^2=1.5\times 10^{-16}](/tpl/images/0048/3070/43bf5.png)
![pH=-log[H^+]=14](/tpl/images/0048/3070/4f928.png)
![[H^+]=10^{-14}](/tpl/images/0048/3070/eec66.png)
![K_w=[H^+][OH^-]=10^{-14}](/tpl/images/0048/3070/f3553.png)
![[OH^-]=\frac{K_w}{[H^+]}=\frac{10^{-14}}{10^{-14}}=1](/tpl/images/0048/3070/8d077.png)
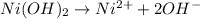
![K_{sp}=[Ni^{2+}][OH^-]^2](/tpl/images/0048/3070/c86a1.png)
![[Ni^{2+}]](/tpl/images/0048/3070/2f342.png) ion.
ion.^2](/tpl/images/0048/3070/f235c.png)
![[Ni^{2+}]=1.5\times 10^{-16}](/tpl/images/0048/3070/989b5.png)
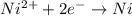
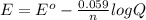
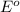 = standard electrode potential of the cell
= standard electrode potential of the cell![E=E^o_{Ni^{2+}/Ni}-\frac{0.059}{n}log\frac{1}{[Ni^{2+}]}](/tpl/images/0048/3070/64772.png)
![E=+0.25V-\frac{0.059}{2}log\frac{1}{[1.5\times 10^{-16}]}](/tpl/images/0048/3070/b7e57.png)


