
Two solutions namely, 500 ml of 0.50 m hcl and 500 ml of 0.50 m naoh at the same temperature of 21.6 are mixed in a constant-pressure calorimeter. the heat capacity of the calorimeter was 450 j/c. given that the specific heat of the solution is 4.184 j/gc, the density of the solution is 1.0 g/ml, and that the heat of neutralization for the process h+oh=h2o is -56.2 kj, what is the final temperature of the mixed solution

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
Two solutions namely, 500 ml of 0.50 m hcl and 500 ml of 0.50 m naoh at the same temperature of 21.6...
Questions


Law, 02.03.2021 01:00


Law, 02.03.2021 01:00




Mathematics, 02.03.2021 01:00


Arts, 02.03.2021 01:00


Mathematics, 02.03.2021 01:00





Spanish, 02.03.2021 01:00

Mathematics, 02.03.2021 01:00

World Languages, 02.03.2021 01:00

Mathematics, 02.03.2021 01:00

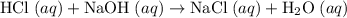
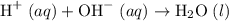
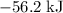 . Thus the combination of every mole of hydrogen ions and hydroxide ions in solution would produce
. Thus the combination of every mole of hydrogen ions and hydroxide ions in solution would produce 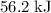 or
or 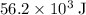 of energy.
of energy.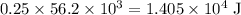 of energy.
of energy. 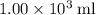 of the 1.0 gram per milliliter solution. Accordingly, it would have a mass of
of the 1.0 gram per milliliter solution. Accordingly, it would have a mass of 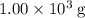 .
. 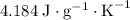 . The solution thus have a heat capacity of
. The solution thus have a heat capacity of 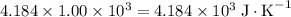 . Note that one degree Kelvins K is equivalent to one degree celsius ℃ in temperature change measurements.
. Note that one degree Kelvins K is equivalent to one degree celsius ℃ in temperature change measurements.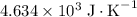 , meaning that its temperature would rise by 1 degree celsius on the absorption of 4.634 × 10³ joules of energy.
, meaning that its temperature would rise by 1 degree celsius on the absorption of 4.634 × 10³ joules of energy.  are available from the reaction. Thus, the temperature of the system shall have risen by 3.03 degrees celsius to 24.6 degrees celsius by the end of the reaction.
are available from the reaction. Thus, the temperature of the system shall have risen by 3.03 degrees celsius to 24.6 degrees celsius by the end of the reaction.

