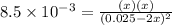Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
For the equilibrium 2 ibr (g) i2 (g) + br2 (g) kp=8.5 ×10-3 at 150 oc. if 0.025 atm of ibr is place...
Questions

English, 09.04.2020 01:41


Chemistry, 09.04.2020 01:41




Mathematics, 09.04.2020 01:41

Mathematics, 09.04.2020 01:41






Law, 09.04.2020 01:41

Mathematics, 09.04.2020 01:41


Mathematics, 09.04.2020 01:41

English, 09.04.2020 01:42


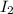 and
and 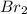 is 0.0211 atm, 0.00195 atm and 0.00195 atm respectively.
is 0.0211 atm, 0.00195 atm and 0.00195 atm respectively.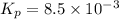
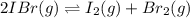
![K_p=\frac{[P_I_2][P_{Br}_2]}{[P_{IBr}]^2}](/tpl/images/0046/9546/983fc.png)