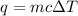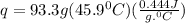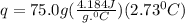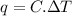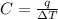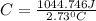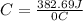Chemistry, 03.07.2019 13:00 xmanavongrove55
Acalorimeter contained 75.0 g of water at 16.95 c. a 93.3-g sample of iron at 65.58 c was placed in it, giving a final temperature of 19.68 c for the system. calculate the heat capacity of the calorimeter. specific heats are 4.184 j/g c for h2o and 0.444 j/g c for fe.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
Acalorimeter contained 75.0 g of water at 16.95 c. a 93.3-g sample of iron at 65.58 c was placed in...
Questions

Mathematics, 09.06.2020 06:57

Chemistry, 09.06.2020 06:57



History, 09.06.2020 06:57


Mathematics, 09.06.2020 06:57

Mathematics, 09.06.2020 06:57

Mathematics, 09.06.2020 06:57






Mathematics, 09.06.2020 06:57

Mathematics, 09.06.2020 06:57

Mathematics, 09.06.2020 06:57



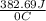 .
. for iron metal = 65.58 - 19.68 = 45.9 degree C
for iron metal = 65.58 - 19.68 = 45.9 degree C