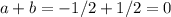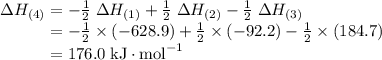Chemistry, 03.07.2019 12:30 adrielalvarez267
Given the following data: n2(g) + 4h2(g) + cl2(g) → 2nh4cl(s) δh = -628.9 kj n2(g) + 3h2(g) → 2nh3(g) δh = -92.2 kj 2hcl(g) → h2(g) + cl2(g) δh = 184.7 kj find the δh of the following reaction: nh4cl(s) → nh3(g) + hcl(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

You know the right answer?
Given the following data: n2(g) + 4h2(g) + cl2(g) → 2nh4cl(s) δh = -628.9 kj n2(g) + 3h2(g) → 2nh3...
Questions


Social Studies, 19.05.2021 05:20



History, 19.05.2021 05:20


Mathematics, 19.05.2021 05:20




Social Studies, 19.05.2021 05:20

Chemistry, 19.05.2021 05:20


Mathematics, 19.05.2021 05:20

Mathematics, 19.05.2021 05:20




English, 19.05.2021 05:20

Advanced Placement (AP), 19.05.2021 05:20

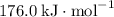
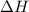 can be determined with the Hess's Law. The key is to find the appropriate coefficient for each of the given equations.
can be determined with the Hess's Law. The key is to find the appropriate coefficient for each of the given equations.  ,
, 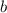 , and
, and  be letters such that
be letters such that 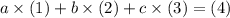 . This relationship shall hold for all chemicals involved.
. This relationship shall hold for all chemicals involved. 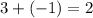 shall resemble the number of
shall resemble the number of 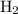 left on the product side when the second equation is directly added to the third. Similarly
left on the product side when the second equation is directly added to the third. Similarly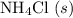 :
: 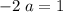
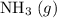 :
: 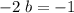
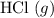 :
: 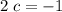
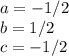 and
and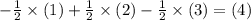
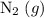 for instance. Nitrogen isn't present in the net equation. The sum of its coefficient shall, therefore, be zero.
for instance. Nitrogen isn't present in the net equation. The sum of its coefficient shall, therefore, be zero.