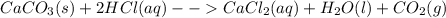The reaction between calcium carbonate (caco3) and hcl produces calcium chloride (cacl2), carbon dioxide (co2), and water (h2o).what happens when the concentration of hydrogen chloride (hcl) molecules is doubled in this reaction? caco3 + 2hcl → cacl2 + co2 + h2o when the hydrogen chloride concentration doubles, the number of collisions between the reactants (increases, decreases, remains constant) , which causes the rate of the forward reaction to (increase, decrease, remain constant)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
The reaction between calcium carbonate (caco3) and hcl produces calcium chloride (cacl2), carbon dio...
Questions

Mathematics, 03.10.2019 05:30

History, 03.10.2019 05:30







Mathematics, 03.10.2019 05:30

History, 03.10.2019 05:30

Social Studies, 03.10.2019 05:30

Business, 03.10.2019 05:30

Computers and Technology, 03.10.2019 05:30

Computers and Technology, 03.10.2019 05:30



History, 03.10.2019 05:30


Biology, 03.10.2019 05:30

Mathematics, 03.10.2019 05:30