
Chemistry, 03.07.2019 11:30 lobatospitones
In this reaction, how does the rate of forward reaction vary with the concentration of the product? 2h2s(g) ⇌ 2h2(g) + s2(g) it increases with an increase in the concentration of s2(g). it decreases with a decrease in the concentration of h2(g). it increases with a decrease in the concentration of h2(g). it decreases with an increase in the concentration of s2(g). it decreases with increase in the concentration of h2(g). multiple answers

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2


Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3

You know the right answer?
In this reaction, how does the rate of forward reaction vary with the concentration of the product?...
Questions


Mathematics, 22.02.2021 23:10


Mathematics, 22.02.2021 23:10

History, 22.02.2021 23:10




Chemistry, 22.02.2021 23:10




Computers and Technology, 22.02.2021 23:10

Social Studies, 22.02.2021 23:10

Mathematics, 22.02.2021 23:10

Geography, 22.02.2021 23:10


English, 22.02.2021 23:10


English, 22.02.2021 23:10

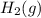 . D) It decreases with increase in the concentration of
. D) It decreases with increase in the concentration of 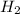 or
or 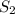 is increased, the reaction will shift in backward direction and the rate of forward direction decreases.
is increased, the reaction will shift in backward direction and the rate of forward direction decreases.

