
Chemistry, 03.07.2019 04:00 guzmangisselle
Why are saturated hydrocarbons generally less reactive than unsaturated hydrocarbons? a. atoms are not easily added to molecules with single bonds. b. atoms are not easily added to molecules with double bonds. c. saturated hydrocarbons have fewer carbon atoms than unsaturated hydrocarbons.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1
You know the right answer?
Why are saturated hydrocarbons generally less reactive than unsaturated hydrocarbons? a. atoms are...
Questions

Arts, 31.05.2021 17:10


English, 31.05.2021 17:10



Mathematics, 31.05.2021 17:10

Chemistry, 31.05.2021 17:10


Mathematics, 31.05.2021 17:10

Mathematics, 31.05.2021 17:10

Mathematics, 31.05.2021 17:10

English, 31.05.2021 17:10

Computers and Technology, 31.05.2021 17:10


Mathematics, 31.05.2021 17:10



Mathematics, 31.05.2021 17:10


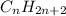
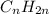 and
and 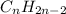
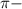 bonds. They are weaker than
bonds. They are weaker than 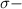 bonds and thus can be easily broken. Addition reactions is more favorable for the compounds having
bonds and thus can be easily broken. Addition reactions is more favorable for the compounds having 

