
Chemistry, 03.07.2019 03:30 trinity7265
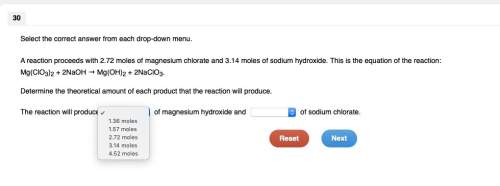
Areaction proceeds with 2.72 moles of magnesium chlorate and 3.14 moles of sodium hydroxide. this is the equation of the reaction: mg(clo3)2 + 2naoh → mg(oh)2 + 2naclo3.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Areaction proceeds with 2.72 moles of magnesium chlorate and 3.14 moles of sodium hydroxide. this is...
Questions

History, 08.11.2019 09:31


Mathematics, 08.11.2019 09:31


History, 08.11.2019 09:31

English, 08.11.2019 09:31



Mathematics, 08.11.2019 09:31

History, 08.11.2019 09:31


Mathematics, 08.11.2019 09:31


Biology, 08.11.2019 09:31

English, 08.11.2019 09:31


Mathematics, 08.11.2019 09:31


Biology, 08.11.2019 09:31



