
Chemistry, 03.07.2019 02:00 brittneyrenae7338
To make use of an ionic hydrate for storing solar energy, you place 510.0 kg of sodium sulfate decahydrate on your house roof. assuming complete reaction and 100% efficiency of heat transfer, how much heat (in kj) is released to your house at night? note that sodium sulfate decahydrate will transfer 354 kj/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
To make use of an ionic hydrate for storing solar energy, you place 510.0 kg of sodium sulfate decah...
Questions

Biology, 09.11.2020 08:10

Biology, 09.11.2020 08:10


Mathematics, 09.11.2020 08:10


Mathematics, 09.11.2020 08:10

Mathematics, 09.11.2020 08:10

Chemistry, 09.11.2020 08:10


Mathematics, 09.11.2020 08:10




History, 09.11.2020 08:10


Biology, 09.11.2020 08:10

Social Studies, 09.11.2020 08:10


English, 09.11.2020 08:10

Mathematics, 09.11.2020 08:10

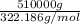 = 1582.936 mol of H₂₀Na₂O₁₄S.
= 1582.936 mol of H₂₀Na₂O₁₄S.

