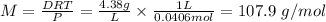Chemistry, 03.07.2019 02:00 ayoismeisalex
Ima chemist found the density of freon-11 (cfcl3) to be 5.58 g/l under her experimental conditions. her measurements showed that the density of an unknown gas was 4.38 g/l under the same conditions. what is the molar mass of the unknown?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1


Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
Ima chemist found the density of freon-11 (cfcl3) to be 5.58 g/l under her experimental conditions....
Questions

Advanced Placement (AP), 10.03.2021 20:40

Mathematics, 10.03.2021 20:40

Mathematics, 10.03.2021 20:40




Physics, 10.03.2021 20:40


Mathematics, 10.03.2021 20:40

Computers and Technology, 10.03.2021 20:40

History, 10.03.2021 20:40


Biology, 10.03.2021 20:40


Mathematics, 10.03.2021 20:40

Mathematics, 10.03.2021 20:40


History, 10.03.2021 20:40


Physics, 10.03.2021 20:40

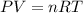 , where P is the pressure, V is the volume, n number of moles, R gas constant and T the temperature in kelvins. As well as the density formula
, where P is the pressure, V is the volume, n number of moles, R gas constant and T the temperature in kelvins. As well as the density formula 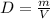 . First we calculate the molarity for Freon
. First we calculate the molarity for Freon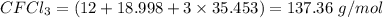 .
.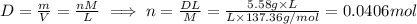
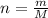 , so we insert this concept to our ideal gas equation
, so we insert this concept to our ideal gas equation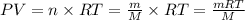 . Since
. Since 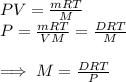 . The measurements take place in same conditions so,
. The measurements take place in same conditions so,