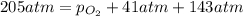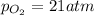Chemistry, 03.07.2019 01:00 FailingstudentXD
Ascuba diver’s air tank contains oxygen, helium, and nitrogen at a total pressure of 205 atmospheres. the partial pressure of nitrogen is 143 atmospheres, and the partial pressure of helium is 41 atmospheres. what is the partial pressure of oxygen in the tank? a. 21 atm b. 103 atm c. 307 atm d. 389 atm

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1


Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
Ascuba diver’s air tank contains oxygen, helium, and nitrogen at a total pressure of 205 atmospheres...
Questions


English, 16.02.2021 16:30




Social Studies, 16.02.2021 16:30

English, 16.02.2021 16:30

Mathematics, 16.02.2021 16:30



Mathematics, 16.02.2021 16:30



Social Studies, 16.02.2021 16:30

English, 16.02.2021 16:30


Mathematics, 16.02.2021 16:30


Chemistry, 16.02.2021 16:30

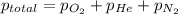
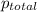 = 205 atm
= 205 atm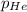 = 41 atm
= 41 atm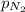 = 143 atm
= 143 atm