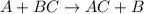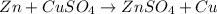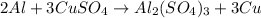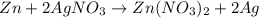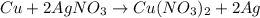30 pts! balancing equations. 2. for part 2: single-displacement reactions: for each of the four single-displacement reactions, describe what happened in each well. if a chemical reaction occurred, write a balanced equation for it. then using the a, b symbols, write a general equation for a single-displacement reaction. a+bc=ac+b here are the chemical formulas of the reactants for each reaction: • zinc – zn copper sulfate – cuso4 zn+cuso4-> cu+znso4 • aluminum – al copper sulfate – cuso4 no reaction • zinc – zn silver nitrate – ag(no3) • copper – cu silver nitrate – ag(no3)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3


Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
30 pts! balancing equations. 2. for part 2: single-displacement reactions: for each of the four s...
Questions


English, 03.12.2021 18:20




English, 03.12.2021 18:20

Mathematics, 03.12.2021 18:20











Chemistry, 03.12.2021 18:20