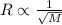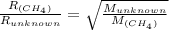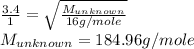Chemistry, 02.07.2019 23:00 timothyashburn8
Explain the relationship between the rate of effusion of a gas and its molar mass. methane gas (ch4) effuses 3.4 times faster than an unknown gas. determine the molar mass of the unknown gas. show your work or explain your answer, giving specific values used to determine the answer.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1


Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Explain the relationship between the rate of effusion of a gas and its molar mass. methane gas (ch4)...
Questions



Chemistry, 20.09.2019 22:10

Mathematics, 20.09.2019 22:10


Mathematics, 20.09.2019 22:20

Mathematics, 20.09.2019 22:20

Chemistry, 20.09.2019 22:20


Mathematics, 20.09.2019 22:20

History, 20.09.2019 22:20

Physics, 20.09.2019 22:20


Biology, 20.09.2019 22:20

Mathematics, 20.09.2019 22:20


Mathematics, 20.09.2019 22:20

Chemistry, 20.09.2019 22:20

Biology, 20.09.2019 22:20

Computers and Technology, 20.09.2019 22:20

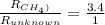
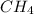 = 16 g/mole
= 16 g/mole