
The molecule carbon disulfide cs2 is nonpolar and has only london dispersion forces between the molecules. carbon tetrachloride ccl4 is also nonploar but is made larger molecules that are more strongly affected by london dispersion forces. based on this information cs2 likely has a melting point and a vapor pressure when compared with ccl4

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
The molecule carbon disulfide cs2 is nonpolar and has only london dispersion forces between the mole...
Questions



Mathematics, 15.06.2021 16:50


Mathematics, 15.06.2021 16:50

Social Studies, 15.06.2021 16:50

Mathematics, 15.06.2021 16:50

English, 15.06.2021 16:50

Mathematics, 15.06.2021 16:50

Mathematics, 15.06.2021 16:50





History, 15.06.2021 16:50

Mathematics, 15.06.2021 16:50

Computers and Technology, 15.06.2021 16:50



Mathematics, 15.06.2021 16:50

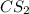 where as the vapor pressure of
where as the vapor pressure of ![CS_2[/tex[ is higher than [tex]CCl_4](/tpl/images/0043/9801/99b5e.png) .
.

