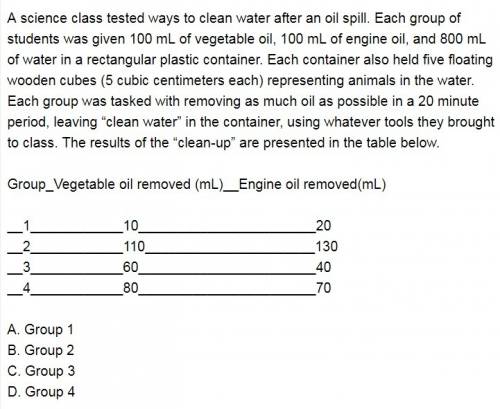
Ascience class tested ways to clean water after an oil spill. each group of students was given 100 ml of vegetable oil, 100 ml of engine oil, and 800 ml of water in a rectangular plastic container. each container also held five floating wooden cubes (5 cubic centimeters each) representing animals in the water. each group was tasked with removing as much oil as possible in a 20 minute period, leaving “clean water” in the container, using whatever tools they brought to class. the results of the “clean-up” are presented in the table below. group vegetable oil removed (ml) engine oil removed (ml) 1 10 20 2 110 130 3 60 40 4 80 70 based on the results above, which group’s data seem unreliable?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1
You know the right answer?
Ascience class tested ways to clean water after an oil spill. each group of students was given 100 m...
Questions


Health, 04.03.2021 22:10

Biology, 04.03.2021 22:10

History, 04.03.2021 22:10






Mathematics, 04.03.2021 22:10




Mathematics, 04.03.2021 22:10


Mathematics, 04.03.2021 22:10

History, 04.03.2021 22:10


Chemistry, 04.03.2021 22:10

Mathematics, 04.03.2021 22:10