
Chemistry, 02.07.2019 12:30 geminigirl077
Ahydrogen-like ion is an ion containing only one electron. the energy of the electron in a hydrogen-like ion is given by en = ? (2.18 × 10? 18j) z2 ( 1 n2 ) where n is the principal quantum number and z is the atomic number of the element. plasma is a state of matter consisting of positive gaseous ions and electrons. in the plasma state, a mercury atom could be stripped of its 80 electrons and therefore could exist as hg80+. use the equation above to calculate the energy required for the last ionization step: hg79+(g) ? hg80+(g)+ e?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Ahydrogen-like ion is an ion containing only one electron. the energy of the electron in a hydrogen-...
Questions

Mathematics, 03.07.2019 03:00

Mathematics, 03.07.2019 03:00

Biology, 03.07.2019 03:00

Mathematics, 03.07.2019 03:00




Biology, 03.07.2019 03:00




History, 03.07.2019 03:00


Mathematics, 03.07.2019 03:00




English, 03.07.2019 03:00

Business, 03.07.2019 03:00

Mathematics, 03.07.2019 03:00


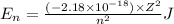
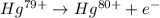
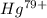 is
is 
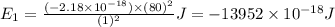
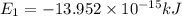 (Conversion Factor: 1kJ = 1000J)
(Conversion Factor: 1kJ = 1000J)

