
Chemistry, 02.07.2019 09:30 alexa006ox9k63
If the density of mercury is 1.36 × 10 by 4 kgm - 3 at 0 degrees. calculate its value at 100 degrees and at 22 degrees. take cubic expansivity of mercury as equal to 180 × 10-6 k - 1

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1
You know the right answer?
If the density of mercury is 1.36 × 10 by 4 kgm - 3 at 0 degrees. calculate its value at 100 degrees...
Questions







Biology, 02.12.2020 19:00



History, 02.12.2020 19:00


SAT, 02.12.2020 19:00








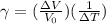 Multiply by V₀ΔT and transpose
Multiply by V₀ΔT and transpose


