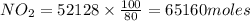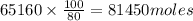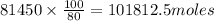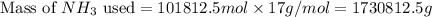Chemistry, 02.07.2019 09:30 hehefjf8610
What mass of nh3 in grams must be used to produce 1.81 tons of hno3 by the ostwald process, assuming an 80.0 percent yield in each step (1 ton=2000 lb; 1 lb= 453.6 g)?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
What mass of nh3 in grams must be used to produce 1.81 tons of hno3 by the ostwald process, assuming...
Questions


Mathematics, 10.12.2020 19:40

Arts, 10.12.2020 19:40






Physics, 10.12.2020 19:40


Geography, 10.12.2020 19:40

Mathematics, 10.12.2020 19:40

Mathematics, 10.12.2020 19:40



Chemistry, 10.12.2020 19:40

Health, 10.12.2020 19:40

Mathematics, 10.12.2020 19:40

Social Studies, 10.12.2020 19:40

Mathematics, 10.12.2020 19:40

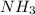 used is 1730812.5 grams.
used is 1730812.5 grams.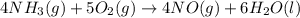
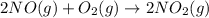
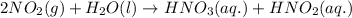
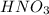 produced = 1.81 tons
produced = 1.81 tons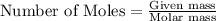 ....(1)
....(1)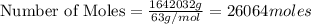
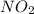 , theorectically (From Step-III) = 2 × 26064 = 52128 moles
, theorectically (From Step-III) = 2 × 26064 = 52128 moles