
Type the correct answer in the box. express the answer to three significant figures. given: ch4 + 2o2 → co2 + 2h2o, δh = -890 kj/mol how much energy is released when 59.7 grams of methane (ch4) reacts with oxygen? the combustion of 59.7 grams of methane releases 34.5 kilojoules of energy.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
Type the correct answer in the box. express the answer to three significant figures. given: ch4 + 2...
Questions


Social Studies, 28.03.2020 01:36


History, 28.03.2020 01:36

History, 28.03.2020 01:36

Mathematics, 28.03.2020 01:37

Mathematics, 28.03.2020 01:37

Mathematics, 28.03.2020 01:37


Mathematics, 28.03.2020 01:37





Mathematics, 28.03.2020 01:37

English, 28.03.2020 01:37

Mathematics, 28.03.2020 01:37



Physics, 28.03.2020 01:37

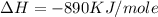
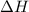 is in negative that means the energy is releasing.
is in negative that means the energy is releasing.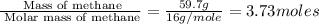
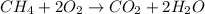
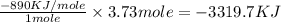 of energy
of energy

