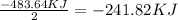Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

You know the right answer?
Water forms according to the equation below: 2h2(g) + o2(g) jpg 2h2o(g) hrxn = -483.64 kj how much...
Questions

History, 03.11.2019 09:31



Mathematics, 03.11.2019 09:31


Computers and Technology, 03.11.2019 09:31

Health, 03.11.2019 09:31

Chemistry, 03.11.2019 09:31




English, 03.11.2019 09:31

Biology, 03.11.2019 09:31

Advanced Placement (AP), 03.11.2019 09:31

Mathematics, 03.11.2019 09:31


Mathematics, 03.11.2019 09:31


English, 03.11.2019 09:31

Mathematics, 03.11.2019 09:31

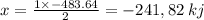

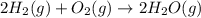
 released amount of energy = -483.64 KJ
released amount of energy = -483.64 KJ