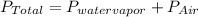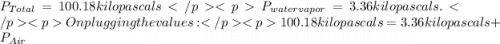Chemistry, 02.07.2019 05:30 heavenwagner
You collect a sample of gases from an indoor pool area. the sample contains air and water vapor. the total pressure is 100.18 kilopascals, and the partial pressure of the water vapor is 3.36 kilopascals. what is the partial pressure of the air in the sample? a. 29.8 kpa b. 51.77 kpa c. 96.82 kpa d. 103.54 kpa e. 337 kpa

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 23.06.2019 13:50
Which compounds are the brønsted-lowry bases in this equilibrium: hc2o4– + h2bo3– h3bo3 + c2o42– ? a. h2bo3– and h3bo3 b. hc2o4– and h3bo3 c. hc2o4– and c2o42– d. h2bo3– and c2o42– e. hc2o4– and h2bo3–
Answers: 2

Chemistry, 23.06.2019 14:30
Among the elements of the main group the first ionization energy increases
Answers: 3
You know the right answer?
You collect a sample of gases from an indoor pool area. the sample contains air and water vapor. the...
Questions

History, 22.09.2019 05:30


Mathematics, 22.09.2019 05:30

History, 22.09.2019 05:30





Mathematics, 22.09.2019 05:30


Mathematics, 22.09.2019 05:30

Mathematics, 22.09.2019 05:30


English, 22.09.2019 05:30

Mathematics, 22.09.2019 05:30


Mathematics, 22.09.2019 05:30

Mathematics, 22.09.2019 05:30