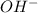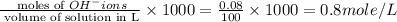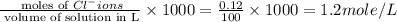Chemistry, 02.07.2019 03:00 stefanylopez731
Calculate the molarity of each type of ion remaining in solution after 20.0 ml of 6.00 m hcl is mixed with 50.0 ml of 2.00 m barium hydroxide and 30.0 ml of water.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3

Chemistry, 23.06.2019 10:00
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1

Chemistry, 23.06.2019 10:30
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1

You know the right answer?
Calculate the molarity of each type of ion remaining in solution after 20.0 ml of 6.00 m hcl is mixe...
Questions








English, 27.06.2019 05:30


Social Studies, 27.06.2019 05:30



Mathematics, 27.06.2019 05:30





Mathematics, 27.06.2019 05:30

History, 27.06.2019 05:30

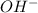 ions = 0.8 mole/L
ions = 0.8 mole/L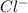 ions = 1.2 mole/L
ions = 1.2 mole/L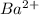 ions = 1 mole/L
ions = 1 mole/L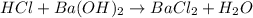
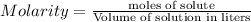
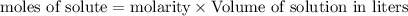
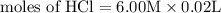 = 0.12
= 0.12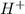 and 1 mole of
and 1 mole of 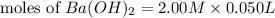 = 0.1
= 0.1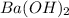 dissociates to give 2 moles of
dissociates to give 2 moles of