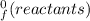Chemistry, 02.07.2019 02:30 hfroslie9840
What is the change in enthalpy for the following reaction? 2h2o2(aq)  2h2o(l) + o2(g) given: h2o: ∆h= -242 kjh2o2: ∆h= -609 kja.-286 kjb. -572 kjc. 572 kjd. 286 kj

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1


You know the right answer?
What is the change in enthalpy for the following reaction? 2h2o2(aq)  2h2o(l)&nb...
Questions




Computers and Technology, 26.06.2020 20:01



Physics, 26.06.2020 20:01

Mathematics, 26.06.2020 20:01

Mathematics, 26.06.2020 20:01



Arts, 26.06.2020 20:01


Mathematics, 26.06.2020 20:01

Mathematics, 26.06.2020 20:01

Mathematics, 26.06.2020 20:01



History, 26.06.2020 20:01

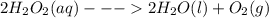
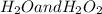 are:
are: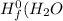 ) = -285.8kJ/mol[/tex]
) = -285.8kJ/mol[/tex]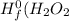 ) = -187.6 kJ/mol[/tex]
) = -187.6 kJ/mol[/tex]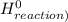 =∑ΔH
=∑ΔH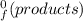 -∑ΔH
-∑ΔH