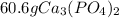Chemistry, 02.07.2019 02:00 fseftrwer2522
Calcium nitrate reacts with sodium phosphate to form sodium nitrate and calcium phosphate. given 96.1 grams of calcium nitrate, what’s the theoretical yield of calcium phosphate? use the periodic table and the polyatomic ion resource. the mass of calcium phosphate the reaction can produce is

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
Calcium nitrate reacts with sodium phosphate to form sodium nitrate and calcium phosphate. given 96....
Questions

Mathematics, 15.10.2019 20:30


Spanish, 15.10.2019 20:30

Mathematics, 15.10.2019 20:30

Chemistry, 15.10.2019 20:30


Social Studies, 15.10.2019 20:30



History, 15.10.2019 20:30


Mathematics, 15.10.2019 20:30



Chemistry, 15.10.2019 20:30


Chemistry, 15.10.2019 20:30

Mathematics, 15.10.2019 20:30