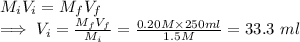Chemistry, 01.07.2019 23:30 leeamation31
You wish to make 250. ml of 0.20 m kcl from a stock solution of 1.5 m kcl and di water. a. indentify each of these quantities from the problem statement: cstock= cdilute= vdilute= b. calculate vstock, the volume of stock solution needed.

Answers: 1
Another question on Chemistry



Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 21.06.2019 22:30
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1
You know the right answer?
You wish to make 250. ml of 0.20 m kcl from a stock solution of 1.5 m kcl and di water. a. indentif...
Questions

Biology, 15.02.2022 04:20





Social Studies, 15.02.2022 04:20

Physics, 15.02.2022 04:20




Social Studies, 15.02.2022 04:20

Mathematics, 15.02.2022 04:20

Mathematics, 15.02.2022 04:20

Business, 15.02.2022 04:20


Mathematics, 15.02.2022 04:20


English, 15.02.2022 04:20


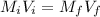 , where
, where 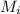 and
and 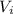 are the stock concentration and volume respectively, then
are the stock concentration and volume respectively, then 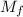 and
and 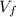 are the dilute concentration and volume respectively.
are the dilute concentration and volume respectively. 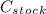 = 1.5 M KCI,
= 1.5 M KCI, 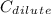 =0.20M KCl,
=0.20M KCl, 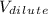 =250ml KCl
=250ml KCl