
Chemistry, 01.07.2019 19:00 gus2006santos
What volume of h2o is formed at stp when 6.0g of al is treated with excess naoh? naoh + al + h2o —-> naal(oh)4 + h2 (g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:50
what is the density of an object that has a mass of 10 g and a volumeof 5 ml? a. 0.5 g/ mlb. 2 g/mlc. 15 g/ mld. 50 g/ ml
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
What volume of h2o is formed at stp when 6.0g of al is treated with excess naoh? naoh + al + h2o —-...
Questions


Chemistry, 03.02.2021 21:40


Mathematics, 03.02.2021 21:40

Chemistry, 03.02.2021 21:40


Mathematics, 03.02.2021 21:40


Mathematics, 03.02.2021 21:40

Computers and Technology, 03.02.2021 21:40


Mathematics, 03.02.2021 21:40


Advanced Placement (AP), 03.02.2021 21:40

English, 03.02.2021 21:40




Mathematics, 03.02.2021 21:40

Mathematics, 03.02.2021 21:40

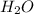 is 14.784 L.
is 14.784 L.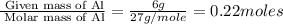
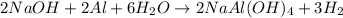
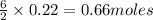 of
of 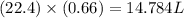 volume of
volume of 

