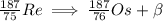Chemistry, 23.09.2019 13:10 golderhadashaowtatz
Chemistry! ! !
1. which of the following solutions would have a ph value greater than 7?
a. [oh-] = 2.4 × 10^-2 m
b. [h3o+] =1.53 × 10^-2 m
c. 0.0001 m hcl
d. [oh^-] = 4.4 × 10^-9 m
2. consider the following equation for an equilibrium system: (attached)
which concentration(s) would be included in the denominator of the equilibrium constant expression?
a. pb(s), co2(g), and so2(g)
b. pbs(s), o2(g), and c(s)
c. o2(g), pb(s), co2(g), and so2(g)
d. o2(g)
3. the oxidation number of the sulfur atom in the so^2- 4 ion is
a. +2.
b. -2.
c. +6.
d. +4.
4. in the following reaction, which is the oxidizing agent? (attached - reaction starts with agno2)
a. agno2
b. cl2
c. koh
d. kcl
5. complete the following nuclear equation (attached w/ choices)
6. which two particles have the same mass but opposite charge?
a. a beta particle and a positron
b. a neutron and a proton
c. a proton and an electron
d. an alpha particle and a proton
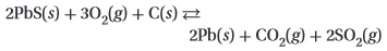
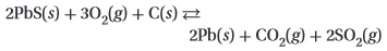

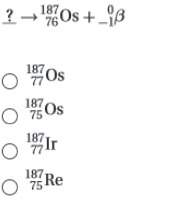

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Chemistry! ! !
1. which of the following solutions would have a ph value greater than 7? <...
1. which of the following solutions would have a ph value greater than 7? <...
Questions


Biology, 07.07.2019 18:50




Mathematics, 07.07.2019 18:50



Mathematics, 07.07.2019 18:50



Computers and Technology, 07.07.2019 18:50

Mathematics, 07.07.2019 18:50


Mathematics, 07.07.2019 18:50


History, 07.07.2019 18:50

Mathematics, 07.07.2019 19:00


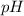 and
and 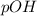 is given by
is given by 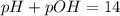 , We can use
, We can use ![pH=-log[H^+]](/tpl/images/0254/9117/15713.png) and
and ![pOH= -log[OH^-]](/tpl/images/0254/9117/291d4.png) . In a we are given the concentration of [OH] and so we use that to find the pOH, then from pOH we can find the pH.
. In a we are given the concentration of [OH] and so we use that to find the pOH, then from pOH we can find the pH.![pOH= -log [2.4 \times 10^-^2] = 1.62\\\\pH = 14 - 1.62= 12.38](/tpl/images/0254/9117/a7174.png)
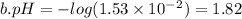
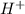 and 0.0001
and 0.0001 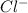 , hence
, hence ![pH= -log[0.0001] = 4](/tpl/images/0254/9117/358f1.png)
![d.pOH= -log [4.4 \times 10^-9] = 8.36\\\\pH= 14- 8.36 = 5.64](/tpl/images/0254/9117/bb698.png)
![a.[OH^-] = 2.4 \times 10^-^2](/tpl/images/0254/9117/6eb94.png) has a pH 12.36 which is greater than 7
has a pH 12.36 which is greater than 7 .
. ![K= \frac{[products]}{[reactants]}](/tpl/images/0254/9117/375cf.png) .
.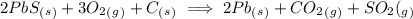
![K= \frac{[CO_2][SO_2]}{[O_2]}](/tpl/images/0254/9117/6da50.png)
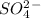 is -2 and in all its compounds oxygen has -2 charge, hence
is -2 and in all its compounds oxygen has -2 charge, hence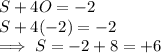
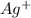 and
and 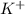 are spectactor ions in this reaction.
are spectactor ions in this reaction.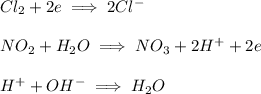
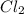 gains electrons to become
gains electrons to become