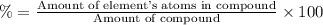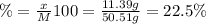Chemistry, 01.07.2019 11:30 cmflores3245
A50.51g sample of a compound made from phosphorus and chlorine is decomposed. analysis of the products showed that 11.39 g of phosphorus atoms were produced. answer using three significant figures. what is the percent by mass of phosphorus? % what is the percent by mass of chlorine? %

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
A50.51g sample of a compound made from phosphorus and chlorine is decomposed. analysis of the produc...
Questions

History, 20.07.2019 16:30

Biology, 20.07.2019 16:30

English, 20.07.2019 16:30





History, 20.07.2019 16:30




Physics, 20.07.2019 16:30