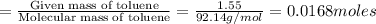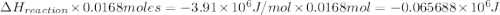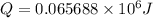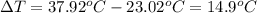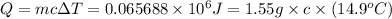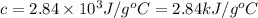Chemistry, 01.07.2019 11:30 samanthashade3434
The combustion of toluene has a δerxn of −3.91 × 103kj/mol. when 1.55 g of toluene (c7h8) undergoes combustion in a bomb calorimeter, the temperature rises from 23.02 ∘c to 37.92 ∘c. find the heat capacity of the bomb calorimeter.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
The combustion of toluene has a δerxn of −3.91 × 103kj/mol. when 1.55 g of toluene (c7h8) undergoes...
Questions

Physics, 03.03.2020 19:22





Mathematics, 03.03.2020 19:22




Mathematics, 03.03.2020 19:22



Mathematics, 03.03.2020 19:22

SAT, 03.03.2020 19:22

History, 03.03.2020 19:22

Mathematics, 03.03.2020 19:22

Mathematics, 03.03.2020 19:22


English, 03.03.2020 19:22

Biology, 03.03.2020 19:22

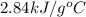 .
.