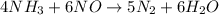For the reaction nh3 + no → n2 + h2o, identify the reactants, products, and their coefficients once the equation is balanced. reactants: 2nh3 and 2no; products: 2n2 and 3h2o products: 2nh3 and 3no; reactants: 2n2 and 3h2o reactants: 4nh3 and 6no; products: 5n2 and 6h2o products: 4nh3 and 2no; reactants: 3n2 and 6h2o

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1


Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
For the reaction nh3 + no → n2 + h2o, identify the reactants, products, and their coefficients once...
Questions


Social Studies, 30.11.2021 20:00

Social Studies, 30.11.2021 20:00




Social Studies, 30.11.2021 20:00


Mathematics, 30.11.2021 20:00

Social Studies, 30.11.2021 20:00

Mathematics, 30.11.2021 20:00

SAT, 30.11.2021 20:00




Social Studies, 30.11.2021 20:00




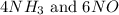
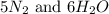
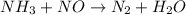
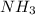 , the coefficient '6' put before the
, the coefficient '6' put before the 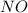 , the coefficient '5' put before the
, the coefficient '5' put before the 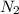 and the coefficient '6' put before the
and the coefficient '6' put before the 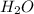 and then we get the balanced chemical equation.
and then we get the balanced chemical equation.