
Chemistry, 01.07.2019 08:30 andrea1704
The formation of cscl from cs(s) and cl2 (g) involves the following steps: cs(s) -> cs(g) 1/2cl2(g) -> cl(g) cs(g) -> cs + (g) + e – cl(g) + e - -> cl - (g) cs+(g) + cl - (g) -> cscl(s) which of these steps absorb energy and which release energy?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
The formation of cscl from cs(s) and cl2 (g) involves the following steps: cs(s) -> cs(g) 1/2...
Questions

English, 18.10.2019 01:30


Mathematics, 18.10.2019 01:30

Mathematics, 18.10.2019 01:30



History, 18.10.2019 01:30

English, 18.10.2019 01:30

Mathematics, 18.10.2019 01:30

Social Studies, 18.10.2019 01:40



Mathematics, 18.10.2019 01:40

Mathematics, 18.10.2019 01:40

Biology, 18.10.2019 01:40

Mathematics, 18.10.2019 01:40

Social Studies, 18.10.2019 01:40

History, 18.10.2019 01:40


Mathematics, 18.10.2019 01:40

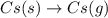
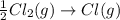
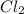 gas in order to break bond between two Cl atoms to form isolated(alone) single chlorine atom.So, the energy will be absorbed by the chlorine gas molecule in this reaction.
gas in order to break bond between two Cl atoms to form isolated(alone) single chlorine atom.So, the energy will be absorbed by the chlorine gas molecule in this reaction.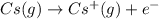
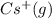 cation. In order to remove an electron from the outer most shell of Cs atom energy will be required by Cs atom, So, energy will be absorbed in this reaction.
cation. In order to remove an electron from the outer most shell of Cs atom energy will be required by Cs atom, So, energy will be absorbed in this reaction.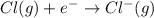
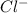 anion from Cl atom in gaseous state. Chlorine atom need one electron to attain noble gas configuration. So, when an electron is added to the outer most shell of chlorine it attains stability of fully filled outermost shell by which it releases energy on addition of an electron.
anion from Cl atom in gaseous state. Chlorine atom need one electron to attain noble gas configuration. So, when an electron is added to the outer most shell of chlorine it attains stability of fully filled outermost shell by which it releases energy on addition of an electron.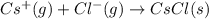
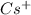 cation and
cation and 

