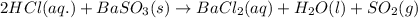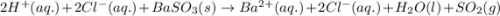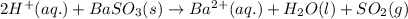Chemistry, 01.07.2019 05:30 claytonp7695
Write a net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and barium sulfite (s) are combined. note: sulfites follow the same solubility trends as sulfates.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
Write a net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and bariu...
Questions


Mathematics, 13.03.2020 07:26

History, 13.03.2020 07:26




Mathematics, 13.03.2020 07:26

Mathematics, 13.03.2020 07:27

History, 13.03.2020 07:28






Mathematics, 13.03.2020 07:30

Mathematics, 13.03.2020 07:31



Mathematics, 13.03.2020 07:31

Mathematics, 13.03.2020 07:31