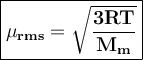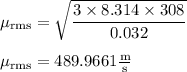Chemistry, 01.07.2019 03:00 smartperraorwhateva
Calculate the rms speed of an oxygen gas molecule, o2, at 35.0 ∘c .express your answer numerically in meters per second.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
Calculate the rms speed of an oxygen gas molecule, o2, at 35.0 ∘c .express your answer numerically i...
Questions

Biology, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30




Chemistry, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30





Biology, 18.03.2021 03:30

History, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30