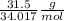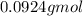Chemistry, 01.07.2019 02:30 katiekellerman9947
If 31.5 grams of hydrogen peroxide reacts to form 16.7 grams of water, how many grams of oxygen must simultaneously be formed?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2


Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
If 31.5 grams of hydrogen peroxide reacts to form 16.7 grams of water, how many grams of oxygen must...
Questions


Mathematics, 20.07.2019 11:00







History, 20.07.2019 11:00


Mathematics, 20.07.2019 11:00

Mathematics, 20.07.2019 11:00

Social Studies, 20.07.2019 11:00

English, 20.07.2019 11:00



Mathematics, 20.07.2019 11:00

Health, 20.07.2019 11:00

History, 20.07.2019 11:00