
Chemistry, 01.07.2019 01:30 iiwolfiexuni
The hydrogen atom is not actually electronegative enough to form bonds to xenon. were the xenon-hydrogen bond to exist, what would be the structure of xeh4? double-click any atom and type xe to change the label. draw the molecule by placing atoms on the grid and connecting them with bonds. show all lone pairs of electrons.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1


Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
The hydrogen atom is not actually electronegative enough to form bonds to xenon. were the xenon-hydr...
Questions


Mathematics, 20.10.2020 22:01

Physics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01


Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Spanish, 20.10.2020 22:01


History, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01


Biology, 20.10.2020 22:01



Chemistry, 20.10.2020 22:01

Chemistry, 20.10.2020 22:01

English, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

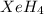 will be square-planar.
will be square-planar.

