Chemistry, 01.07.2019 00:00 lalala1212
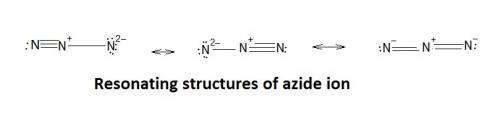
The azide ion, n−3, is a symmetrical ion, all of whose contributing structures have formal charges. draw three important contributing structures for this ion. draw the molecule by placing atoms on the grid and connecting them with bonds. include all nonbonding electrons. show the formal charges of all atoms.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
The azide ion, n−3, is a symmetrical ion, all of whose contributing structures have formal charges....
Questions

Computers and Technology, 14.12.2021 17:20



Biology, 14.12.2021 17:20

SAT, 14.12.2021 17:20






English, 14.12.2021 17:20

Computers and Technology, 14.12.2021 17:20

Law, 14.12.2021 17:20

Mathematics, 14.12.2021 17:20

English, 14.12.2021 17:20

English, 14.12.2021 17:20

History, 14.12.2021 17:20

Spanish, 14.12.2021 17:20

Computers and Technology, 14.12.2021 17:20

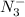 , is a symmetrical ion, all of whose contributing structures have formal charges.
, is a symmetrical ion, all of whose contributing structures have formal charges.